

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Ionization energies and electron affinities
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
Organic Chemistry
الجزء والصفحة:
P23
11-2-2016
2731
Ionization energies and electron affinities
Ionization energies
The ionization energy of hydrogen
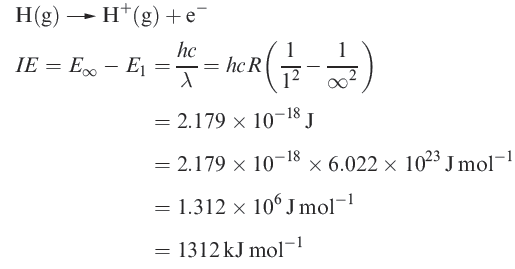
H atom has only one electron, no additional ionization processes can occur. For multi-electron atoms, successive ionizations are possible.
The first ionization energy, IE1, of an atom is the internal energy change at 0 K, Δ U(0 K), associated with the removal of the first valence electron (equation 1.17); the energy change is defined for a gas phase process. The units are kJ mol-1 or electron volts (eV).

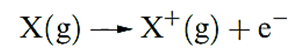 ( 1.1 )
( 1.1 )
It is often necessary to incorporate ionization energies into thermochemical calculations (e.g. Born–Haber or Hess cycles) and it is convenient to define an associated enthalpy change, ΔH(298K).
Since the difference between ΔH(298K) and ΔU(0K) is very small , values of IE can be used in thermochemical cycles so long as extremely accurate answers are not required.

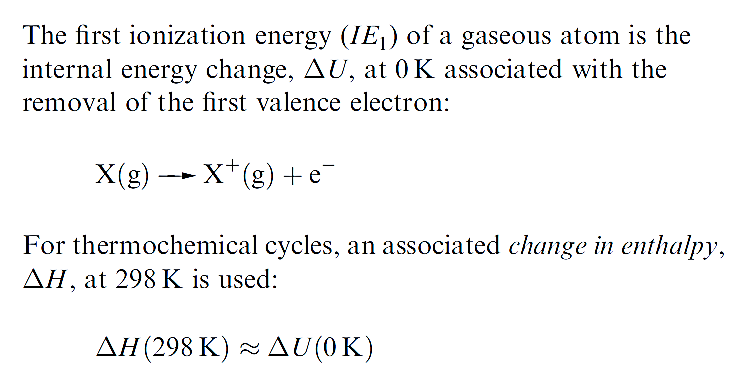
The second ionization energy, IE2, of an atom refers to step 1.2; note that this is equivalent to the first ionization of the ion X+. Equation 1.2 describes the step corresponding to the third ionization energy, IE3, of X, and successive ionizations are similarly defined:

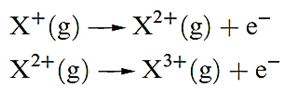 (1.2)
(1.2)
Figure 1. shows the variation in the values of IE1 as a function of Z. Several repeating patterns are apparent and some features to note are:
- the high values of IE1 associated with the noble gases;
- the very low values of IE1 associated with the group 1 elements;
- the general increase in values of IE1 as a given period is crossed;
- the discontinuity in values of IE1 on going from an element in group 15 to its neighbour in group 16;
- the decrease in values of IE1 on going from an element in group 2 or 12 to its neighbour in group 13.
- the rather similar values of IE1 for a given row of d-block elements.
Each of these trends can be rationalized in terms of ground state electronic configurations. The noble gases (except for He) possess ns2 np6 configurations which are particularly stable and removal of an electron requires a great deal of energy. The ionization of a group 1 element involves loss of an electron from a singly occupied ns orbital with the resultant X ion possessing a noble gas configuration.
The general increase in IE1 across a given period is a consequence of an increase in Zeff. A group 15 element has a ground state electronic configuration ns2np3 and the np level is half-occupied. A certain stability is associated with such configurations and it is more difficult to ionize a group 15 element than its group 16 neighbour. In going from Be (group 2) to B (group 13), there is a marked decrease in IE1 and this may be attributed to the relative stability of the filled shell 2s2 configuration compared with the 2s2 2p1 arrangement; similarly, in going from Zn (group 12) to Ga (group 13), we need to consider the difference between 4s2 3d10 and 4s2 3d10 4p1 configurations.
The noble gases (except for He) possess ns2 np6 configurations which are particularly stable and removal of an electron requires a great deal of energy. The ionization of a group 1 element involves loss of an electron from a singly occupied ns orbital with the resultant X ion possessing a noble gas configuration.
The general increase in IE1 across a given period is a consequence of an increase in Zeff. A group 15 element has a ground state electronic configuration ns2 np3 and the np level is half-occupied. A certain stability is associated with such configurations and it is more difficult to ionize a group 15 element than its group 16 neighbour. In oing from Be (group 2) to B (group 13), there is a marked decrease in IE1 and this may be attributed to the relative stability of the filled shell 2s2 configuration compared with the 2s2 2p1 arrangement; similarly, in going from Zn (group 12) to Ga (group 13), we need to consider the difference between 4s2 3d10 and 4s2 3d10 4p1 configurations.
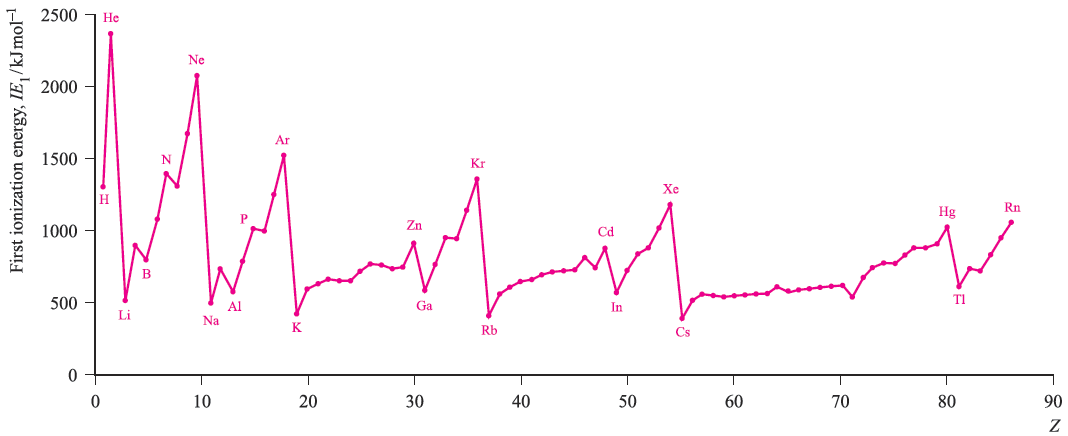
Fig. 1. The values of the first ionization energies of the elements up to Rn.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















