x

هدف البحث
بحث في العناوين
بحث في اسماء الكتب
بحث في اسماء المؤلفين

اختر القسم
موافق


النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الحيوية

مواضيع عامة في المضادات الحيوية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Yeast Prions Show Unusual Inheritance
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المصدر:
LEWIN’S GENES XII
الجزء والصفحة:
15-6-2021
1819
Yeast Prions Show Unusual Inheritance
KEY CONCEPTS
- The Sup35 protein in its wild-type soluble form is a termination factor for translation.
- Sup35 can also exist in an alternative form of oligomeric aggregates, in which it is not active in protein synthesis.
- The presence of the oligomeric form causes newly synthesized protein to acquire the inactive structure.
- Conversion between the two forms is influenced by chaperones.
- The wild-type form has the recessive genetic state psi- and the mutant form has the dominant genetic state PSI+ .
One of the clearest cases of the dependence of epigenetic inheritance on the condition of a protein is provided by the behavior of prions. They have been characterized in two circumstances: (1) by genetic effects in yeast and (2) as the causative agents of neurological diseases in mammals, including humans. A striking epigenetic effect is found in yeast, where two different states can be inherited that map to a single genetic locus, though the sequence of the gene is the same in both states. The two different states are [psi- ] and [PSI+ ]. A switch in condition occurs at a low frequency as the result of a spontaneous transition between the states.
The [psi] genotype maps to the locus SUP35, which codes for a translation termination factor. FIGURE 27.15 shows the effects of the Sup35 protein in yeast. In wild-type cells, which are characterized as [psi -], the gene is active, and the Sup35 protein terminates protein synthesis. In cells of the mutant [PSI+ ] type, the oligomerized factor does not function, which causes a failure of proper termination of protein synthesis. (This was originally detected by the lethal effects of the enhanced efficiency of suppressors of ochre codons in [PSI+] strains.)
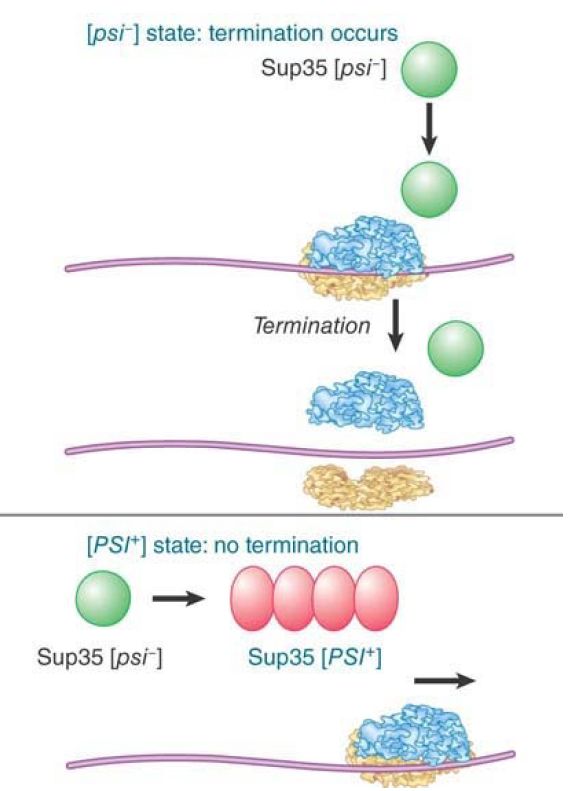
FIGURE 1. The state of the Sup35 protein determines whether termination of translation occurs.
[PSI+ ] strains have unusual genetic properties. When a [psi- ] strain is crossed with a [PSI+ ] strain, all of the progeny are [PSI+ ]. This is a pattern of inheritance that would be expected of an extrachromosomal agent, but the [PSI+ ] trait cannot be mapped to any such nucleic acid. The [PSI+ ] trait is metastable, which means that, though it is inherited by most progeny, it is lost at a higher rate than is consistent with mutation. Similar behavior also is shown by the locus URE2, which encodes a protein required for nitrogenmediated repression of certain catabolic enzymes. When a yeast strain is converted into an alternative state called [URE3], the Ure2 protein is no longer functional.
The [PSI+ ] state is determined by the conformation of the Sup35 protein. In a wild-type [psi- ] cell, the protein displays its normal function. In a [PSI+ ] cell, though, the protein is present in an alternative conformation in which its normal function has been lost. To explain the unilateral dominance of [PSI+ ] over [psi -] in genetic crosses, we must suppose that the presence of protein in the [PSI+ ] state causes all the protein in the cell to enter this state.
This requires an interaction between the [PSI+ ] protein and newly synthesized protein, which probably reflects the generation of an oligomeric state in which the [PSI+ ] protein has a nucleating role, as illustrated in FIGURE 2.
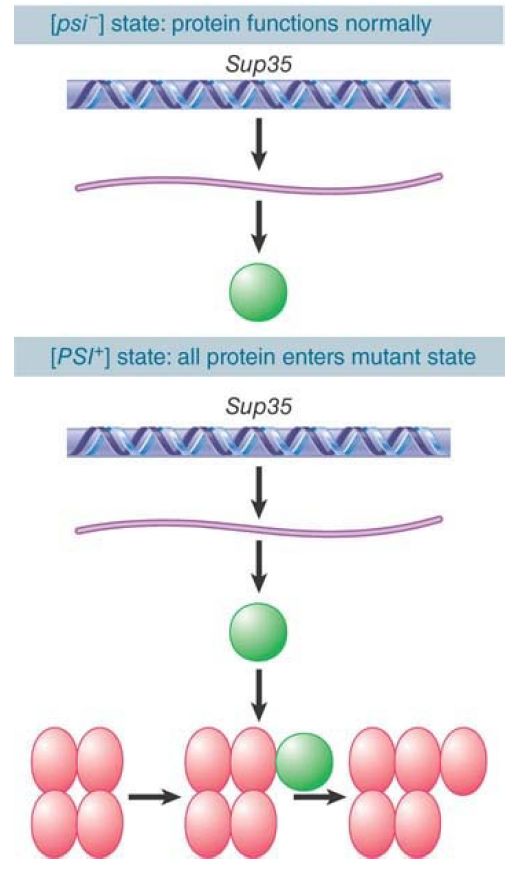
FIGURE 2. Newly synthesized Sup35 protein is converted into the [PSI+ ] state by the presence of preexisting [PSI+ ] protein.
A feature common to both the Sup35 and Ure2 proteins is that each consists of two domains that function independently. The Cterminal domain is sufficient for the activity of the protein. The Nterminal domain is sufficient for formation of the structures that make the protein inactive. Thus, yeast in which the N-terminal domain of Sup35 has been deleted cannot acquire the [PSI+ ] state, and the presence of a [PSI+ ] N-terminal domain is sufficient to maintain Sup35 protein in the [PSI+ ] condition. The critical feature of the N-terminal domain is that it is rich in glutamine and asparagine residues.
Loss of function in the [PSI+ ] state is due to the sequestration of the protein in an oligomeric complex. Sup35 protein in [PSI+ ] cells is clustered in discrete foci, whereas the protein in [psi- ] cells is diffused in the cytosol. Sup35 protein from [PSI+ ] cells forms amyloid fibers in vitro—these have a characteristic high content of β-sheet structures. These amyloid fibers consist of a parallel inregister β-sheet structure, which allows the prion amyloid to induce a “templating” action at the end of filaments. This templating action provides the faithful transmission of variant differences in these molecules and allows self-reproduction encoding heritable information reminiscent of the behavior of genes.
The involvement of protein conformation (rather than covalentmod ification) is suggested by the effects of conditions that affect protein structure. Denaturing treatments cause loss of the [PSI+ ] state. In particular, the chaperone Hsp104 is involved in inheritance of [PSI+ ]. Its effects are paradoxical. Deletion of HSP104 prevents maintenance of the [PSI+ ] state, and overexpression of Hsp104 also causes loss of the [PSI+ ] state through elimination of Sup35 proteins. The Ssa and Ssb components of the Hsp70 chaperone system affect Sup35 prion genesis directly through cooperation with Hsp104. Ssa and Ssb binding is facilitated by Hsp40 chaperones through interactions with Sup35 oligomers. At high concentrations, Hsp104 eliminates Sup35 prions while low levels of Hsp104 stimulate prion genesis and alleviate some Hsp70–Hsp40 pairs. Thus, the interplay among Hsp104, Hsp70, and Hsp40 regulates the formation, growth, and elimination of Sup35 prions.
Using the ability of Sup35 to form the inactive structure in vitro, it is possible to provide biochemical proof for the role of the protein. FIGURE 3 illustrates a striking experiment in which the protein was converted to the inactive form in vitro, put into liposomes (where in effect the protein is surrounded by an artificial membrane), and then introduced directly into cells by fusing the liposomes with [psi- ] yeast. The yeast cells were converted to [PSI+ ]! This experiment refutes all of the objections that were raised to the conclusion that the protein has the ability to confer the epigenetic state. Experiments in which cells are mated, or in which extracts are taken from one cell to treat another cell, always are susceptible to the possibility that a nucleic acid has been transferred. When the protein by itself does not convert target cells, but the protein converted to the inactive state can do so, the only difference is the treatment of the protein—which must therefore be responsible for the conversion.
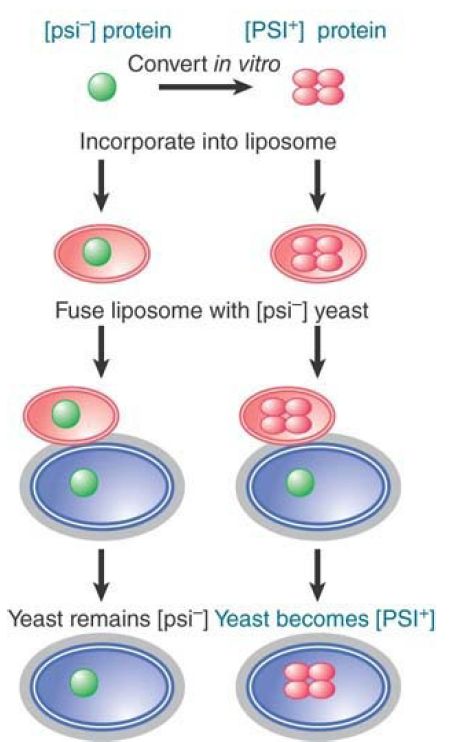
FIGURE 3. Purified protein can convert the [psi- ] state of yeast to [PSI+ ].
The ability of yeast to form the [PSI ] prion state depends on the yeast’s genetic background. The yeast must be [PIN ] in order for the [PSI ] state to form. The [PIN ] condition itself is an epigenetic state. It can be created by the formation of prions from any one of several different proteins. These proteins share a key characteristic of Sup35, which is that they have Gln/Asn-rich domains. Overexpression of these domains in yeast stimulates formation of the [PSI ] state. This suggests that there is a common model for the formation of the prion state that How does the presence of one Gln/Asn protein influence the formation of prions by another? We know that the formation of Sup35 prions is specific to Sup35 protein; that is, it does not occur by cross-aggregation with other proteins. This suggests that the yeast cell may contain soluble proteins that antagonize prion formation. These proteins are not specific for any one prion. As a
result, the introduction of any Gln/Asn-domain protein that interacts with these proteins will reduce the concentration. This will allow other Gln/Asn proteins to aggregate more easily.
Prions have recently been linked to chromatin-remodeling factors. Swi1 is a subunit of the SWI/SNF chromatin-remodeling complex (see the Eukaryotic Transcription Regulation chapter), and this protein can become a prion. Swi1 aggregates in [SWI ] cells but not in nonprion cells, and is dominantly and cytoplasmically transmitted. This suggests that inheritance through proteins can impact chromatin remodeling and potentially affect gene regulation throughout the genome.