

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Part 1: Readying the HPLC for Operation : Experimental Procedure
المؤلف:
LibreTexts Project
المصدر:
................
الجزء والصفحة:
.................
18-4-2020
1872
1. Ready the HPLC by making sure the solvent reservoirs are full and the waste bottles have at least 1 Liter of volume available to accommodate the waste solvent.
2. Launch Open Lab by clicking HPLC1 (online) (Figure 1.1)

Figure 1.1: HPLC1 (online) desktop icon.
3. Use the Instrument Control Tab. Confirm that all components are green and indicate “ready.” (Figure 1.2)
a. If this is not the case, press the “On” button.
b. The DAD will take a few minutes to warm up; if there is a lightning bolt through the purple lamp, wait until it has gone away, and the ready bar has turned green.
c. The fraction collector will not be used in this experiment.
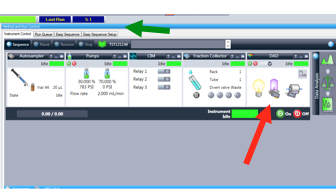
Figure 1.2: "Instrument Control" tab indicted by top, green arrow. Bottom right, red arrow indicates the DAD window.
Parabens are a class of chemicals widely used as preservatives in the cosmetic and pharmaceutical industries. Parabens are effective preservatives and are primarily used for their bactericidal and fungicidal properties. Parabens can be found in shampoos, shaving gels, personal lubricants, topical/parenteral pharmaceuticals, spray tanning lotion and toothpaste. Parabens can also be used as food additives. Recently, there has been an in-vivo and in-vitro studies have shown that parabens have weak estrogenic activity. Because parabens are endocrine disrupters, paraben concentrations have been mitigated and must not surpass the maximum permitted levels. Paraben mixtures are often used in order to increase the efficiency of the preservation. Take the time to look up general paraben structures to develop an understanding of their chemical structure.
Because parabens are often found in mixtures, HPLC can be used to separate the individual compounds. To determine the components in the mixture, reverse phase HPLC will be performed through a C18 column. In this portion of the experiment, gradient and isocratic elution data will be collected and compared. The paraben mixture has already been prepared for you and contains 4-hydroxy benzoic acid, methyl-4-hydroxy benzoate, ethyl-4-hydroxy benzoate, and propyl-4-hydroxy benzoate.
To begin, five isocratic experiments will be performed. The conditions for the five experiments are listed in Table 2.2.
Table 2.2: Isocratic Experiment Conditions
| Experimental Run | Mobile Phase Composition |
|---|---|
| 1 | 100 % methanol |
| 2 | 90 % methanol / 10 % water |
| 3 | 80 % methanol / 20 % water |
| 4 | 70 % methanol / 30 % water |
| 5 | 60 % methanol / 40 % water |
1. Set up six methods. One for each of the 5 isocratic runs and one for the gradient run.**Your TA will be assisting you while you set up your sequence.**
2. Select “PARABENS.S” in the “sequence” menu bar.
3. Go to “sequence” and click “sequence template” and see the order of the samples. Each run will use the same sample which should be vial slot 1.
4. Each method should be different for each run. To check this, click the “method” menu bar, click “method” in the selection menu, and select “edit entire method.”
5. Look through the method with your TA and check that all the parameters are correct. DO NOT change anything in the method unless you confirm with your TA that you are doing so. Save the method once you are done.
6. Confirm that you have enough of the paraben mixture in the sample vial and that the sample vial is in vial slot 1.
7. Click “run sequence.”
8. You can print the reports after each run or after all runs are complete. You can find the data reports by clicking the “data analysis” tab in the bottom.
9. Before moving on, confirm that you have peaks for each of your runs. If you notice any issues with your data, talk with your TA.
*The Paraben mixture runs will take approximately one hour to run, so the caffeine standards and the beverage samples can be prepared while they are running*