

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Carbon-dioxide (CO2) and Carbon-monoxide (CO) Lasers
المؤلف:
LibreTexts Project
المصدر:
................
الجزء والصفحة:
.................
28-2-2020
1868
Carbon-dioxide (CO2) and Carbon-monoxide (CO) Lasers
In both of these lasers the gaseous medium is made-up of molecules, which in addition to electronic energy levels of atoms also have both molecular vibrational and rotational energy levels. The vibrational energy levels are similar to finer spaced ladder rungs that span two rungs of the electronic energy levels. The rotational levels are still more finely spaced rungs that span the vibrational rungs! In these gas lasers the lasing transitions occur among the vibrational levels, typically belonging to different electronic levels.
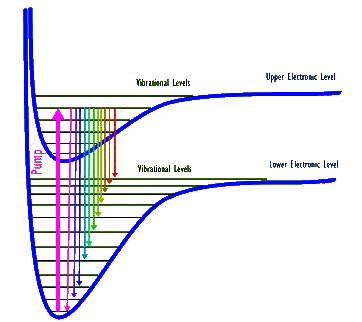
Above diagram shows two electronic and several of their associated vibrational levels for a hypothetical molecule. (Electronic levels are shown as "bent rungs" because in the molecule atoms can change their separation distance and therefore their electronic energy. Also, note that rotational levels are not shown.) A thick arrow depicts a pump that excites the molecule from its lowest vibrational level belonging to the lower electronic level to the 5th highest vibrational level of the next upper electronic level. The excited molecule can then de-excite out of this upper level into many possible vibrational levels. Each one of these de-excitations produces a photon whose energy, and therefore its wavelength, corresponds to that specific de-excitation. As a result, when a collection of these molecules are all excited by this pump they generate a number (eleven, in this drawing) of different wavelength photons.
Specifically, CO2
lasers can generate an output wavelength from about 9 micro-meters (mm, or microns) to about 11 microns (1 micron is one millionth of a meter, or 1000 nm.) These outputs generally contain many closely spaced wavelengths, if the laser is used for high power output. But for more wavelength specific applications the optical cavity of the laser is designed to amplify just one or a few of the vibrational radiative decay lines. The wavelength range for the CO laser is lower, from about 5 to 6 mm. Another feature of these gas lasers that make them one of the most versatile of all gas lasers is that they can be made to operate over a large range of power outputs, either in a pulsed or cw mode. The CO2
laser, in particular, ranges in cw power from few Watts to kWs, making these lasers ideal for many industrial applications including welding and drilling.
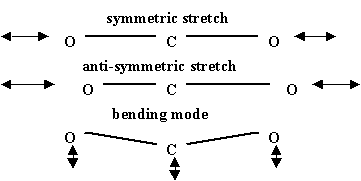
Molecular vibrations for CO2
, a linear molecule, are shown in the figure below. Other combinations of these are possible but these three are fundamental. There are different varieties of CO2
lasers that flow fresh gases through the resonant cavity area in order both to remove heat and to provide lots of gas to achieve high laser powers. For these lasers in the cw mode powers can reach as high as 100 kW. These intense laser beams are essentially tremendous invisible "heat" beams that can cut through thick pieces of metal and are used extensively in industrial applications.
We mention two interesting tidbits about these lasers. First, since glass in not very transparent to IR light, the mirrors are actually made of special crystalline materials that are transparent to the IR. Second, recall that IR light is invisible to our eyes and so special precautions are needed to protect people working around these lasers. It turns out that although these lasers can easily cut through metal, they cannot pass through a thin sheet of clear plexiglass, and so often these systems are housed in a plexiglass shell to block any stray reflected IR light.
Other types of gas lasers include the nitrogen laser (N2), excimers, copper-vapor, gold-vapor, and chemical lasers. Of these the excimer lasers and the chemical lasers are the most different from the ones we have already discussed above.
 الاكثر قراءة في التحليل الآلي (الطيفي)
الاكثر قراءة في التحليل الآلي (الطيفي)
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















