
Applications of AMS
 المؤلف:
LibreTexts Project
المؤلف:
LibreTexts Project
 المصدر:
................
المصدر:
................
 الجزء والصفحة:
.................
الجزء والصفحة:
.................
 18-2-2020
18-2-2020
 1553
1553
Applications of AMS
Common radioisotope elements measured with AMS and their applications are shown in Table 1, below. Because 14C analysis is by far the most popular application of AMS, the methods discussed below are all techniques used involving 14C.
Table 1. Radioisotope elements generally measured with AMS and their applications.
| Element (Common Isotope) |
Radioisotope with AMS |
Natural abundance |
Half-life (yr) |
Study application |
| Hydrogen (1H) |
3H |
trace |
12.33 |
Biological/biomedical Nutritional trace |
| Beryllium (9Be) |
10Be |
trace |
1,510,000 |
Geochronology
Hydrogeological study
Exposure dating |
| Carbon (12C) |
14C |
1 x10-10% |
5730 |
Biological/biomedical
Nutritional trace |
| Aluminum (27Al) |
26Al |
trace, synthetic |
720,000 |
Biological/biomedical Exposure dating |
| Chlorine (35Cl) |
36Cl |
7x10−11% |
301,000 |
Earth Science Hydrogeological study Exposure dating Migration of nuclear waste |
| Calcium (40Ca) |
41Ca |
trace, synthetic |
116,000 |
Biological/biomedical
Nuclear weapon testing |
| Nickel (58Ni) |
59Ni |
trace, synthetic |
112,000 |
Nutritional trace |
| Iodine (127I) |
129I |
trace, synthetic |
15,700,000 |
Biological/biomedical
Migration of nuclear waste
Environmental study |
Radiocarbon dating is an analytical method based on the rate of decay of 14C, a radioactive carbon isotope formed in the atmosphere by the reaction between neutrons from cosmic rays and 14N (neutron + 14N = 14C + proton).[2] Resultant 14C atoms are taken up by plants in the form of 14CO2, then transferred to animals though the food chain. When animals and plants die, they cease to uptake 14C, and a steady decay of 14C continues in their tissues over time. 14C atoms decay via electron emission (β radiation) to form 14N, a process which has a half life of 5,730 years. Radiocarbon levels in the atmosphere change according to complex patterns which are affected by a variety of fluctuations ranging from the sun’s solar activity and the earth’s magnetic field, to ocean ventilation rate and climate. 14C analysis of tree rings, corals, lake sediments, ice cores, and other sources has led to a detailed record of 14C variations through time, allowing researchers to establish an official radiocarbon calibration curve (also referred to as a radiocarbon clock) dating back 26,000 calendar years. In the 1960s, nuclear weapons testing released large amounts of neutrons into the atmosphere, nearly doubling 14C activity. Samples taken after this time period can be radiocarbon dated using a 14C bomb curve like the peak shown below in Figure 3, can retrieve very precise dates (within 1 year at the steepest part of the curve).
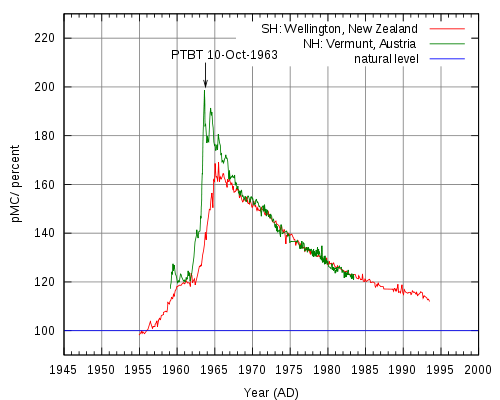
Figure 1. The New Zealand curve (red) is representative of atmospheric 14C in the Southern Hemisphere, and the Austrian curve is representative of the Northern Hemisphere.
14C analysis provides valuable information in the radiocarbon dating of the world’s most priceless artifacts. One such example of the monumental impact of 14C AMS is the radiocarbon dating of the Dead Sea Scrolls to dates from 300 BC to AD 61 by labs in Zurich and Arizona. AMS has also contributed greatly to environmental and atmospheric studies by providing information regarding particle composition and origin. In the biochemical field, synthesized 14C labeled compounds can be administered as a tracer dose for in-vivo human metabolic and drug studies which require AMS analysis of graphitized biological samples.
AMS is a highly sensitive method for isotopic analysis that has numerous key applications that are only growing with advances in technology. High costs and technical complexities that arise with the use of a particle accelerator are the only limits to the widespread use of AMS. Recent times have seen the emergence of commercially available compact accelerators that use as low as 200 kV for radiocarbon dating and biomedical applications, and as particle accelerators become more commonplace, modifications to the instrument have also broadened the number of isotopes the instrument can measure.
 الاكثر قراءة في التحليل الآلي (الطيفي)
الاكثر قراءة في التحليل الآلي (الطيفي)
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


