


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 3-1-2017
Date: 9-2-2018
Date: 6-7-2017
|
Using the crisscross rule
A quick way to determine the formula of an ionic compound is to use the crisscross rule: Take the numerical value of the metal ion’s superscript (forget about the charge symbol) and move it to the bottom right-hand side of the nonmetal’s symbol — as a subscript. Then take the numerical value of the nonmetal’s superscript and make it the subscript of the metal. (Note that if the numerical value is 1, it’s just understood and not shown.) So what happens if you react aluminum and oxygen? Figure 1.1 shows the crisscross rule for this reaction. You get Al2O3.
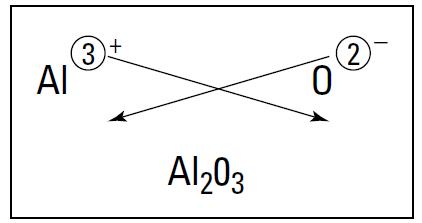
Figure 1.1: Figuring out the formula of aluminum oxide.
Compounds involving polyatomic ions work in exactly the same way. For example, here’s the compound made from the ammonium cation (NH4+) and the sulfide anion (S2–): (NH4)2S
Notice that because you need two ammonium ions (two positive charges) to neutralize the two negative charges of the sulfide ion, you enclose the ammonium ion in parentheses and add a subscript 2. After you use the crisscross rule, reduce all the subscripts by a common factor, if possible, to get the right formula. For example, suppose that you want to write the compound formed when calcium reacts with oxygen. Calcium, an alkaline earth metal, forms a 2+ cation, and oxygen forms a 2– anion. So you may predict that the formula is Mg2O2 But you need to divide each subscript by 2 to get the correct formula: MgO



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|